Amongst all gases only Hydrogen can be solved in Aluminium. Compared with the solubility of gases in iron alloys, however, the quantity is rather low.
The solubility of Hydrogen in Aluminium depends on the content of alloys and on the temperature. The solved quantity furthermore depends on the availability of Hydrogen, which is usually given as the partial pressure and indicated in Millilitres of the solved gas per 100 Grams of metal. (1013 mbar and 0° Celsius; 1 ppm = 1,1124 ml/100g)
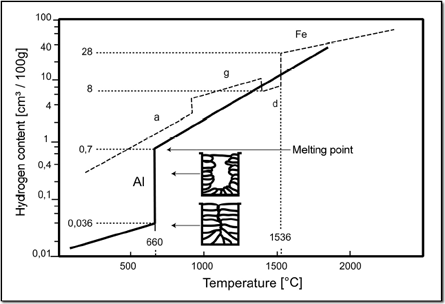
As the solubility of Hydrogen in Aluminium suddenly decreases at a temperature of approx. 600° Celsius during cooling it often comes to porosity caused by frozen gas bubbles. With pure Aluminium the tendency to porosity is most serious, whereas it is lower with alloys. This is due to a smaller leap in the solubility of Hydrogen.
The basic solution to this problem is to keep the level of available Hydrogen as low as possible.
Also the atmospheric influences of humidity and temperature may significantly change the conditions of fabrication during the seasons. Especially when base materials or filler metals are moved from a cold warehouse into a heated fabrication hall it may come to condensation of water on the surface. This massively increases the possibility of porosity.
To get an idea about the possibility of condensation we have developed our dew point calculator. Simply enter the values for air temperature, metal temperature and the relative humidity and you get a definite answer if welding is permitted or not.
Make the following entries:
Possible Countermeasurements:
- Store welding parts and filler metals in the same room for several hours
- Preheat work pieces
- Store filler metals in heated room (30 - 40°C)





